no is odd electron molecule|6.5: Exceptions to the Octet Rule : Baguio There are actually very few stable molecules with odd numbers of electrons that exist, since that unpaired electron is willing to react with other unpaired . 97402, OR home for sale. Gorgeous brand new modern farmhouse on almost 5 acres just a stone's throw from Fern Ridge Reservoir. This "legacy" estate was the original Huey family homestead and current owners demolished old house and built new, while keeping and enhancing the outbuildings and landscape/hardscape, .Harem Hotel takes place on the continent of Syl'anar, a diverse elvish land conquered by devout humans over 300 years ago. This deeply imperfect and technologically advanced world hides many secrets to be .
PH0 · Which among the following is an odd electron molecule?
PH1 · Odd
PH2 · Exceptions to the Octet Rule
PH3 · 9.11: Exceptions to the Octet Rule
PH4 · 66 Violations of the Octet Rule
PH5 · 6.5: Exceptions to the Octet Rule
PH6 · 11.3 Lewis Symbols and Structures
PH7 · 10.5: Exceptions to the Octet Rule
PHJOY Casino is dedicated to offering our players extraordinary bonus rewards. We stand committed to excellence, ensuring our bonuses are among the most lucrative in the gaming industry. Our wide range of promotions includes everything from captivating welcome bonuses to rich cashback offers and special rewards, all crafted to provide maximum .
no is odd electron molecule*******There are actually very few stable molecules with odd numbers of electrons that exist, since that unpaired electron is willing to react with other unpaired .Solution. The correct option is D. all of these. The explanation for correct option. (D) all of these. Odd electron molecules are molecules whose total number of valence electrons is an odd number. The number of valence electrons in NO is 5 + 6 = 11. The number of .
Odd-electron Molecules. We call molecules that contain an odd number of electrons free radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in .
Odd-Electron Molecules. There are a number of molecules whose total number of valence electrons is an odd number. It is not possible for all of the atoms in . Odd-electron Molecules. We call molecules that contain an odd number of electrons free radicals. Nitric oxide, NO, is an example of an odd-electron molecule; .
Having an odd number of electrons in a molecule guarantees that it does not follow the octet rule, because the rule requires eight electrons (or two for hydrogen) around each .
Odd-electron Molecules. We call molecules that contain an odd number of electrons free radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when .Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when oxygen and nitrogen react at high temperatures. To draw the Lewis structure for an odd-electron .
no is odd electron moleculeWith an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Examples of stable odd-electron molecules are NO, NO 2, and ClO 2. The Lewis electron dot diagram for NO is as .
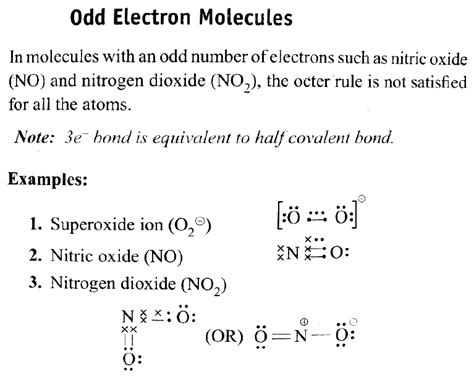
An odd electron molecule among the following is : View Solution. Q3. Number of non-polar molecules among the following is x and number of planar molecules is y. What is x+y? B F 3, C O 2, S O 2, P C I 5, C I F 3, N H 3, C H 4. View Solution. Q4. Among the following the total number of polar molecules are_____. No formal charge at all is the most ideal situation. An example of a stable molecule with an odd number of valence electrons would be nitric oxide. nitric oxide has 11 valence electrons. If you need more information about formal charges, see .Describe being odd electron molecule, NO is colourless. Explain. 02:52. Nitric oxide, though an odd electron molecule, is diamagenetic in liqu. 01:12. Assertion: Nitric oxide is paramagnetic in the liquid and solid states. 02:14. Match {:(A,"Molecules with electron deficient central atoms",1,PCl(5. To draw the Lewis structure for an odd-electron molecule like NO, we follow the same five steps we would for other molecules, but with a few minor changes: Determine the total number of valence (outer shell) electrons. The sum of the valence electrons is 5 (from N) + 6 (from O) = 11. The odd number immediately tells us that we . (B) NO and H 2 SO 4 (A) BCl 3 → Even Electron molecule . SF 6 → Expanded octet molecule (B) NO → Odd Electron molecule . H 2 SO 4 → Expanded octet. (C) SF 6 → Even Electron molecule . H 2 SO 4 → Expanded octet. (D) BCl 3 → Even Electron molecule . NO → Odd Electron molecule. S → 12e-in outer orbit.Odd-electron Molecules. We call molecules that contain an odd number of electrons free radicals. Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when oxygen and nitrogen react at high temperatures. To draw the Lewis structure for an odd-electron molecule like NO, we follow the same five .
To draw the Lewis structure for an odd-electron molecule like NO, we follow the same five steps we would for other molecules, but with a few minor changes: Determine the total number of valence (outer shell) electrons. The sum of the valence electrons is 5 (from N) + 6 (from O) = 11. The odd number immediately tells us that we have a free .6.5: Exceptions to the Octet RuleTo draw the Lewis structure for an odd-electron molecule like NO, we follow the same five steps we would for other molecules, but with a few minor changes: Determine the total number of valence (outer shell) electrons. The sum of the valence electrons is 5 (from N) + 6 (from O) = 11. The odd number immediately tells us that we have a free .
As we stated earlier, molecular orbital theory can therefore explain the bonding in molecules with an odd number of electrons, such as NO, whereas Lewis electron structures cannot. Figure \(\PageIndex{9}\): Molecular Orbital Energy-Level Diagram for NO. Because NO has 11 valence electrons, it is paramagnetic, with a single electron . To draw the Lewis structure for an odd-electron molecule like NO, we follow the same five steps we would for other molecules, but with a few minor changes: Determine the total number of valence (outer shell) electrons. The sum of the valence electrons is 5 (from N) + 6 (from O) = 11. The odd number immediately tells us that we . Odd-Electron Molecules. There are a number of molecules whose total number of valence electrons is an odd number. It is not possible for all of the atoms in such a molecule to satisfy the octet rule. An example is nitrogen dioxide \(\left( \ce{NO_2} \right)\). Each oxygen atom contributes six valence electrons and the nitrogen atom .

To draw the Lewis structure for an odd-electron molecule like NO, we follow the same five steps we would for other molecules, but with a few minor changes: Determine the total number of valence (outer shell) electrons. The sum of the valence electrons is 5 (from N) + 6 (from O) = 11. The odd number immediately tells us that we have a free . Odd-electron molecules. Although they are few, some stable compounds, often called free radicals, have an odd number of electrons in their valence shells. With an odd number of electrons, at least one atom in the molecule will have to violate the octet rule. Examples of stable, odd-electron molecules are \(\ce{NO}\), \(\ce{NO2}\), and . Nitric oxide, NO, is an example of an odd-electron molecule; it is produced in internal combustion engines when oxygen and nitrogen react at high temperatures. To draw the Lewis structure for an odd-electron molecule like NO, we follow the same five steps we would for other molecules, but with a few minor changes: Determine the total .
The hydroxyl radical can also be represented as •OH, with the dot next to the oxygen representing the odd electron. Similar to nitrogen in the NO 2 molecule, oxygen is only surrounded by seven valence electrons. Since the hydroxyl radical will be far more stable if it obtains an eighth electron, it is very reactive.To draw the Lewis structure for an odd-electron molecule like NO, we follow the same five steps we would for other molecules, but with a few minor changes: Determine the total number of valence (outer shell) electrons. The sum of the valence electrons is 5 (from N) + 6 (from O) = 11. The odd number immediately tells us that we have a free .no is odd electron molecule 6.5: Exceptions to the Octet Rule To draw the Lewis structure for an odd-electron molecule like NO, we follow the same five steps we would for other molecules, but with a few minor changes: Determine the total number of valence (outer shell) electrons. The sum of the valence electrons is 5 (from N) + 6 (from O) = 11. The odd number immediately tells us that we . To draw the Lewis structure for an odd-electron molecule like NO, we follow the same five steps we would for other molecules, but with a few minor changes: Determine the total number of valence (outer shell) electrons. The sum of the valence electrons is 5 (from N) + 6 (from O) = 11. The odd number immediately tells us that we .
Assertion: N O2 and CO2 both odd electron molecules and hence dimerizes. Reason :- On dimerisation, N O2 is converted to stable N 2O4 molecule with even number of electrons. Q. An oxide of chlorine which is an odd electron molecule is : Q.
Siargao Bleu Resort and Spa . Siargao Bleu Resort and Spa is a plush beachfront resort that is just a few meters from the famous Cloud 9 Boardwalk. This upscale resort offers classy rooms with wi-fi, flat-screen TV, a coffee maker, .
no is odd electron molecule|6.5: Exceptions to the Octet Rule